Nobel for finding the perfect molecular click
The Nobel Prize in Chemistry, awarded on Wednesday, honours three scientists “for the development of click chemistry and bio-orthogonal chemistry”. These innovative chemistries provide efficient ways to build new molecules that have significant uses, particularly in pharmaceuticals.
In pharmaceuticals, building new molecules is often key to the development of new drugs. For a chemist, the challenge is not only to construct molecules that carry out useful functions, but also to get there using efficient processes.
The Nobel Prize in Chemistry, awarded on Wednesday, honours three scientists “for the development of click chemistry and bio-orthogonal chemistry”. These innovative chemistries provide efficient ways to build new molecules that have significant uses, particularly in pharmaceuticals.
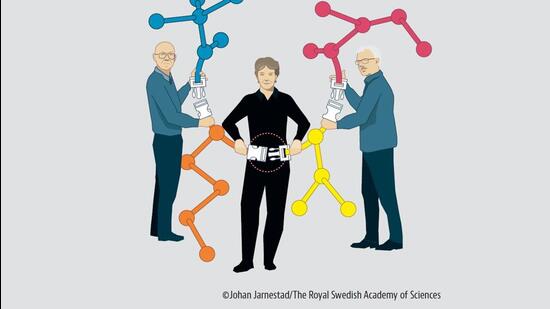
Barry Sharpless of Scripps Research, who had also won the Chemistry Prize in 2001, won his second for his work on getting molecules to bond in a “click reaction” – they “click” together like two Lego pieces.
Morten Meldal (University of Copenhagen) discovered a reaction that can be utilised to trigger click reactions between numerous different molecules.
And Carolyn R Bertozzi (Stanford University) utilised Sharpless and Meldal’s breakthroughs to develop “bio-orthogonal reactions”. These click reactions work inside a living cell, laying the framework for targeted treatments.
Click chemistry
Sharpless, who became the second scientist to win two Chemistry Nobels (Frederick Sanger won in 1958 and 1980), coined the term “click chemistry” and described it in a paper in 2001.
Click chemistry overcomes a challenge that often faces other methods to create molecules. Many chemists have sought to imitate nature, artificially constructing useful molecules present in plants and animals. While natural substances have successfully inspired many pharmaceuticals, the challenge lies in the various unwanted by-products created in the course of building such molecules. Removing these by-products often leads to loss of material, time and money.
In his paper, Sharpless argued against imitating natural molecules. One difficulty is that bio-molecules are built on bonding between carbon atoms, which is not easy to replicate in the lab. Sharpless suggested that chemists work with simple molecules that already had a complete carbon frame, and link these via nitrogen atoms or oxygen atoms, instead of connecting the carbon atoms themselves. This would prevent many of the side reactions, with a minimal loss of material.
Key click reaction
Independently of each other, Sharpless and Meldal discovered how to make click reactions happen. Meldal’s find happened by accident. While trying to get two compounds to react, Meldal and colleagues in Denmark found that a reaction did happen, but not the targeted one.
The result provided the pathway: Two classes of compounds, called alkynes and azides, could be made to react and form useful chemical structures, called triazoles. Triazoles are a useful product, used in some pharmaceuticals, dyes and agricultural chemicals.
The fact that these two compound classes could react to form triazoles was already known. Previous efforts, however, had led to unwanted byproducts. Meldal’s chance discovery overcame this obstacle. If copper ions are added to the reaction, there are no byproducts, and only one substance is formed. Such a reaction can be used to bond together numerous different molecules.
In 2002, Meldal and Sharpless published separate articles on copper-aided click reactions.
Biorthogonal chemistry
The events leading to Bertozzi’s foray into click chemistry begin in the 1990s, when she began to study a group of molecules. Called glycans, these molecules are often found on the surface of proteins and cells, and play an important role in many biological processes.
At a time when not enough tools existed to study glycans, Bertozzi devised her own process. She used a chemical handle that would map the glycans, and attached a fluorescent molecule to the handle so that she could study the hidden glycans under the emitted light.
The challenge, again, was to avoid unwanted reactions. The chemical handle must react with only the fluorescent molecule, and nothing else. She termed this a “biorthogonal reaction”.
As it turned out, the optimal chemical handle she found was an azide, one of the components in Sharpless and Meldal’s click reaction.
Utilising this idea, however, presented another challenge. The reaction described by Meldal and Sharpless required copper ions, and copper is toxic to living things. Bertozzi found a way out: there was published literature describing a way to get azides and alkynes (the two components in the click reaction) to react without using copper. In 2004, she published the copper-free click reaction, and then demonstrated that it can be used to track glycans.
What next
Bertozzi and other researchers have used these reactions to explore molecular interactions in cells and to study disease processes. For example, Bertozzi found that on the surface of tumour cells, some glycans shut down the immune cells. To break down this defence, she and her colleagues have created a new type of drug, which is now in clinical trials on people with advanced cancer.
Advances in click chemistry, meanwhile, have made it possible to assemble large “libraries” of potential drug molecules, to select for the optimal molecule, an article in Scripps Research Magazine said. Libraries of molecules that could potentially inhibit HIV have been made, and also of potential drugs for diabetes, Alzheimer’s, Parkinson’s, cancer and many other diseases.
All Access.
One Subscription.
Get 360° coverage—from daily headlines
to 100 year archives.


HT App & Website